"Generic 20mg zhewitra with mastercard, medicine remix".
By: Q. Dolok, MD
Clinical Director, Chicago Medical School of Rosalind Franklin University of Medicine and Science
Monographs and treatment summaries are divided into chapters based on specific aspects of medical care georges marvellous medicine order 20mg zhewitra amex, such as Chapter 5 treatment yeast infection cheap 20mg zhewitra free shipping, Infections medicine game purchase 20 mg zhewitra visa, or Chapter 16 medicine quinine 20 mg zhewitra fast delivery, Emergency treatment of poisoning; or drug use related to a particular system of the body, such as Chapter 2, Cardiovascular. Within each therapeutic use, the drugs are organised alphabetically by classification. Antimuscarinics, Beta2-agonist bronchodilators) and then alphabetically within each classification. Appendices, covering interactions, borderline substances, and cautionary and advisory labels. Yellow cards are also included, to facilitate the reporting of adverse events, as well as quick reference guides for life support and key drug doses in medical emergencies, for ease of access. Once in a chapter, location is guided by the side of the page showing the chapter number (the thumbnail), alongside the chapter title. The top of the page includes the therapeutic use (the running head) alongside the page number. Once on a page, visual cues aid navigation: treatment summary information is in black type, with therapeutic use titles similarly styled in black, whereas the use of colour indicates drug-related information, including drug classification titles, drug class monographs, and drug monographs. Although navigation is possible by browsing, primarily access to the information is via the index, which covers the titles of drug class monographs, drug monographs and treatment summaries. The index also includes the names of branded medicines and other topics of relevance, such as abbreviations, guidance sections, tables, and images. Doses for children can be identified by the relevant age range and may vary according to their age or body-weight. However, the monograph in chapter 1 contained only the dose and some selected safety precautions. Now, all of the information for the systemic use of a drug is contained within one monograph, so codeine phosphate p. This carries the advantage of providing all of the information in one place, so the user does not need to flick back and forth across several pages to find all of the relevant information for that drug. Cross references are included in chapter 1, where the management of diarrhoea is discussed, to the drug monograph to assist navigation. Where drugs have systemic and local uses, for example, chloramphenicol, and the considerations around drug use are markedly different according to the route of administration, the monograph is split, as with earlier editions, into the relevant chapters. This means that the majority of drugs are still placed in the same chapters and sections as earlier editions, and although there may be some variation in order, all of the relevant information will be easier to locate. One of the most significant changes to the monograph structure is the increased granularity, with a move from around 9 sections to over 20 sections; sections are only included when relevant information has been identified. Monographs Overview Selecting the dose the dose of a drug may vary according to different indications, routes of administration, age, body-weight, and body surface area. The right dose should be selected for the right age and body-weight (or body surface area) of the child, as well as for the right indication, route of administration, and preparation. For clarity and to aid selection of the correct dose, wherever possible these age and weight ranges now do not overlap. When interpreting age ranges it is important to understand that a child is considered to be 11 up until the point of their 12th birthday, meaning that an age range of child 12 to 17 years is applicable to a child from the day of their 12th birthday until the day before their 18th birthday. Similarly, when interpreting weight ranges, it should be understood that a weight of up to 30 kg is applicable to a child up to , but not including, the point that they tip the scales at 30 kg and a weight range of 35 to 59 kg is applicable to a child as soon as they tip the scales at 35 kg right up until, but not including, the point that they tip the scales at 60 kg. For example, a 3 week old baby born at 27 weeks gestation is treated as having a corrected gestational age of 30 weeks. However, the degree of prematurity, the maturity of renal and hepatic function, and the clinical properties of the drug need to be considered on an individual basis. The calculated dose should not normally exceed the maximum recommended dose for an adult. Calculation by bodyweight in the overweight child may result in much higher doses being administered than necessary; in such cases, the dose should be calculated from an ideal weight for height. Relevant synonyms are included below the title and, in some instances a brief description of the drug action is included. Over future editions these drug action statements will be rolled out for all drugs. In some monographs, immediately below the nomenclature or drug action, there are a number of cross references used to signpost the user to any additional information they need to consider about a drug. This is most common for drugs formulated in combinations, where users will be signposted to the monographs for the individual ingredients. Therefore, indication and dose information has been promoted to the top of the monograph and highlighted by a coloured panel to aid quick reference.
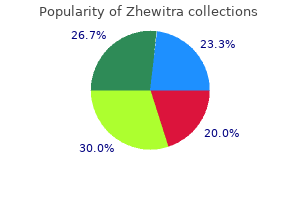
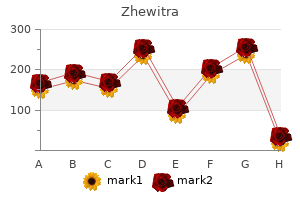
For purposes of assigning Air-Ground Radiotelephone Service licenses through competitive bidding medicine app cheap 20 mg zhewitra otc, the Commission has defined "small business" as an entity that symptoms for mono buy 20 mg zhewitra visa, together with controlling interests and affiliates medicine world nashua nh buy zhewitra mastercard, has average annual gross revenues for the preceding three years not exceeding $40 million medicine gustav klimt purchase zhewitra online now. On June 2, 2006, the auction closed with two winning bidders winning two Air-Ground Radiotelephone Services licenses. Census Bureau data for 2012 show that there were 967 firms that operated for the entire year. Michaels, Deputy Chief, Auctions and Spectrum Access Division, Wireless Telecommunications Bureau, Federal Communications Commission (filed Sept. For purposes of the auction, the Commission defined a "small" business as an entity that, together with controlling interests and affiliates, has average gross revenues for the preceding three years not to exceed $15 million dollars. Census Bureau data in this industry for 2012 show that there were 967 firms that operated for the entire year. It is our understanding that Teligent and its related companies have less than 1,500 employees, though this may change in the future. Wired Telecommunications Carriers are comprised of establishments primarily engaged in operating and/or providing access to transmission facilities and infrastructure that they own and/or lease for the transmission of voice, data, text, sound, and video using wired telecommunications networks. Census Bureau data for 2012 show that there were 3,117 firms that operated that year. The Commission estimates that of these 2,206 licenses, the majority are held by non-profit educational institutions and school districts, which are by statute defined as small businesses. This Economic Census category "comprises establishments primarily engaged in broadcasting images together with sound. The Commission has estimated the number of licensed commercial television stations to be 1,387. In addition, the Commission has estimated the number of licensed noncommercial educational television stations to be 395. We note, however, that in assessing whether a business concern qualifies as "small" under the above definition, business (control) affiliations212 must be included. Our estimate, therefore, likely overstates the number of small entities that might be affected by our action, because the revenue figure on which it is based does not include or aggregate revenues from affiliated companies. In addition, another element of the definition of "small business" requires that an entity not be dominant in its field of operation. We are unable at this time to define or quantify the criteria that would establish whether a specific television broadcast station is dominant in its field of operation. Accordingly, the estimate of small businesses to which rules may apply does not exclude any television station from the definition of a small business on this basis and is therefore possibly over-inclusive. Also, as noted above, an additional element of the definition of "small business" is that the entity must be independently owned and operated. The Commission notes that it is difficult at times to assess these criteria in the context of media entities and its estimates of small businesses to which they apply may be over-inclusive to this extent. This Economic Census category "comprises establishments primarily engaged in broadcasting aural programs by radio to the public. We note, however, that in assessing whether a business concern qualifies as small under the above size standard, business affiliations must be included. It does not matter whether control is exercised, so long as the power to control exists. Census Bureau data for 2012 show that 2,849 radio station firms operated during that year. Census Bureau data for Radio Stations and Television Broadcasting, the Commission estimates that the majority of Auxiliary, Special Broadcast and Other Program Distribution Services firms are small. We also recognize that most commercial translators and boosters are owned by a parent station which, in some cases, would be covered by the revenue definition of a small business entity discussed above. It defined a very small business as an entity with average annual gross revenues not exceeding $3 million for the preceding three years; a small business as an entity with average annual gross revenues not exceeding $15 million for the preceding three years; and an entrepreneur as an entity with average annual gross revenues not exceeding $40 million for the preceding three years. Eight of the ten winning bidders claimed small business status and won 144 of the licenses. These licensees are held by individuals in a noncommercial capacity; these licensees are not small entities. Personal radio services provide short-range, low-power radio for personal communications, radio signaling, and business communications not provided for in other services. Personal radio services include services operating in spectrum licensed under Part 95 of our rules.
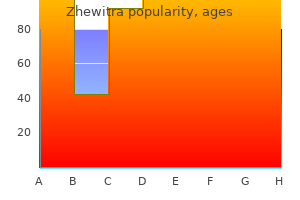
I Add I Foreign Priority Information: this section allows for the applicant to claim priority to a foreign application medications used to treat depression order generic zhewitra canada. Prefix D Middle Name Given Name Family Name Suffix Mailing Address Information: Address 1 Address 2 City Country ii Phone Number Email Address State/Province Postal Code Fax Number Additional Applicant Data may be generated within this form by selecting the Add button medications dictionary order 20mg zhewitra otc. Patent and Trademark Office may not be able to process and/or examine your submission treatment research institute cheap 20mg zhewitra overnight delivery, which may result in termination of proceedings or abandonment of the application or expiration of the patent medicine information zhewitra 20mg free shipping. A record in this system of records may be disclosed, as a routine use, to a Member of Congress submitting a request involving an individual, to whom the record pertains, when the individual has requested assistance from the Member with respect to the subject matter of the record. A record in this system of records may be disclosed, as a routine use, to a contractor of the Agency having need for the information in order to perform a contract. A record from this system of records may be disclosed, as a routine use, to the public after either publication of the application pursuan to 35 U. The application rnust contain three (3) or fevver independent claims and twenty (20) or fevver total c! Other attachments: - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Signature /Brent A. The application must contain three (3) or fewer independent claims and twenty (20) or fewer total claims. The request for a first action interview must include a statement that appiicant agrees not to file a request for a refund of the search fee and any excess claims fees paid in the application after the mailing or notification of the Pre-Interview Communication. Accordingly, pursuant to the requirements of the Act, please be advised that: (1} the general authority for the coliection of t~1is information is 35 U. Patent and Trademark Office is to process and/or examine your submission related to a patent appiication or patent. The information provided by you in this form wili be subject to tile following routine uses: 1. The information on this form will be treated confidentially to the extent allowed under the Freedom of Information Act (5 U. Records from this system of records may be disdosed to the Department of Justice to determine whether disclosure of these records is required by the Freedom of information Act. A record related to an internationai Application fiied under the Patent Cooperation Treaty in this systern of records may be disclosed, as a routine use, to the International Bureau of the World Intellectual Property Organization, pursuant to tile Patent Cooperation Treaty. By filing this request: Applicant is agreeing to make an election without traverse if the Office determines that the daims are not obviously directed to a single invention; and Applicant is agreeing not to request for a refund of the search fee and any excess claims fee paid in the application after the mailing 01· notification of the pre-interview communication prepared by the examiner. A record from this system of records may be disclosed, as a routine use, in the course of presenting evidence to a couti, magistrate, or administrative tribunai, including disciosures to opposing counsel in the course of settlement negotiations. A record in this system of records may be disclosed, as a routine use, to another federal agency tor purposes of National Security review (35 U. Aii st,3tenwnts rnadB herein of my/our own knowledge are true, all statements rn,3dB herein on information and belief are believed to be true, and further that these staternenls were rnacie with the knowledge that wmful false statements and the likE! C, 1001, and may jeopardize thei validity of t11e application or any patent issuing thereon, I hereby acknowledge that any willful faise slaterm~nt made in t~1is declaration is punishable under 18 I! L 93-579} requires that you be given cE,rtain information in connection with your submission of the attached form reiated to a patent application or patent. Accordingly, pursuant to the requirements of the Act, please be advised that: (1) the general authority for the collection of this information is 3fi U. Patent and Trademark Office is to process and/or exarnine your submission related to a patent application or patent If you do not furnish the requested information, the U. Patent and Trademark Office may not be able to process and/or examine your submission, which may resu! The information provided by you in this form will be subject to the following rouUne uses: 1. The information on this form will be treated confidentiaHy to the extent allowed under the Freedom of lnforn1;;ition Act (5 U. Records from this system of records may be disclosed lo lr;e Di:ipartment of Justice to determine whether disclosure of these records is required by the Freedom of Information Act. A record in this systern of records may be clisclosecl, as a routine use, to a Member of Congress submitting a request involving an individual, to whom the rE,cord pertains, when the individual has requested assistance from the Member with respect to the subject matter of the record. A record related to an int,~mational Application filed under the Patent Cooperation Treaty in this system of records may b; disclosed, as a routine use, to the lntemationai Bureau of the Worid inte!!
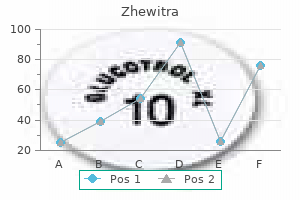
Syndromes
- Damage to your kidneys, liver, or other organs from anti-rejection medications
- Inflammation of tissues that line the wall of the abdomen and cover the abdominal organs
- Dry or inflamed eyes
- Hemangioma
- Drugs
- Do you dye your hair?
- Is irritable
- Muscular twitching
The individual in crisis is assisted to be in the least restrictive setting to resolve the crisis medications with weight loss side effect order zhewitra 20mg on line, whether or not they are committed 94 medications that can cause glaucoma order 20 mg zhewitra free shipping. Clinicians recognize and seek to carefully balance potential conflict between beneficence and personal autonomy medicine 906 generic zhewitra 20mg without a prescription. Due process protections are understood and employed at every level for the person medicine 360 discount zhewitra line. Restrictive interventions such as seclusion and restraint are used as a last resort to ensure safety, and explicit procedures are developed to minimize their use. The assessment and commitment process allows for care transitions into less restrictive levels of care and supports transitions into and out of settings. Trauma history is taken into consideration as part of a thorough assessment while minimizing the risk of re-triggering trauma. Collateral information from family and others who have knowledge of the person is collected and informs the outcome. Clarity and purpose of commitment is carefully reviewed and considered including what services are provided and the criteria for the commitment to be ended. Persons involved in the commitment process are free of material conflict of interest. Practical Tools to Assist Policy Makers in Evaluating, Reforming, and Implementing Involuntary Civil Commitment Taking account of the competing interests at stake in civil commitment, and considering the inherent ethical concerns, this final part of the report offers two practical tools to assist policy makers and others responsible for reforming or implementing civil commitment laws or systems: first, a list of ten general guidelines with which to align commitment policy and practice; and second, a checklist of specific model requirements for inpatient and outpatient commitment statutes. Civil commitment, whether inpatient or outpatient, should be reserved for those reliably diagnosed with a serious mental illness for which there is available treatment that is likely to be effective. If the person is willing and able to engage with services voluntarily, he or she should not be committed. In deciding whether to order commitment, courts should consider the preferences of the person and the degree to which the person understands the nature of his or her mental illness and the likely effect of treatment. A person should not be subject to inpatient commitment unless, without a hospitallevel of care, the person will be at significant risk, in the foreseeable future, of behaving in a way, actively or passively, that brings harm to the person or others. Unless the serious mental illness for which treatment is needed places the person at risk for harm, inpatient commitment should not be used. If an individual is at risk for injury, illness, death, or other major loss solely due to mental illness symptoms such as an inability to exercise self-control, judgment, and discretion in the conduct of his or her daily activities, or to satisfy his or her need for nourishment, personal or medical care, shelter, or self-protection and safety, he or she should be committable. If a less restrictive alternative to inpatient commitment is available, including outpatient commitment, inpatient commitment should not be ordered. If, with the help of family, friends, or others who are available and willing to help, a person is capable of remaining in the community without presenting risks associated with need for treatment, he or she should not be subject to inpatient commitment. A person should not be subject to outpatient commitment unless (i) he or she meets the standard for inpatient commitment, but may be served in a less restrictive setting, or (ii) without the treatment proposed, and other supports the court might order, it is reasonably predictable that the person will experience further disability or deterioration to a degree that, in the foreseeable future, the person will meet the inpatient commitment standard. Legal proceedings should accord due process protection, including prompt notice of rights, assignment of counsel, and an opportunity to challenge commitment before a judge or other judicial authority without unreasonable delay. If law enforcement agencies are responsible for transporting individuals proposed for or under order of commitment, they should assign plainclothes officers in unmarked cars, whenever possible. Shackles and other restraints should be used only if necessary, never as a matter of routine. Unless already incarcerated for a criminal offense, or facing criminal charges, no candidate for commitment should be detained in a jail or other correctional facility pending commitment, and no person who has been committed should be placed in a correctional facility for treatment services. Civil commitment should never be used solely for preventive detention or community control. No court should insist that a hospital or other treatment provider retain an individual in services at a level of care that the hospital or provider believes is unnecessary. Requirements for Civil Commitment: A Checklist for Policy Makers and Practitioners All Civil Commitments (Inpatient or Outpatient) the individual is reliably diagnosed with a serious mental illness. A reasonable effort has been made to help the individual understand the nature of his or her mental illness and the treatment proposed, including the potential risks and benefits of such treatment and the expectable consequences if he or she is or is not committed. Inpatient Commitments Without commitment, and as a result of the serious mental illness diagnosed, the individual will be at significant risk, in the foreseeable future, of behaving in a way, actively or passively.
Purchase zhewitra 20mg free shipping. What are Flu Symptoms? | Baby Care Basics | Parents.