"Trusted 300 mg zyloprim, treatment yellow fever".
By: K. Dan, M.B. B.CH. B.A.O., Ph.D.
Medical Instructor, University of Houston
Depending on the situation parts of the plan can be activated and deactivated as necessary medications hard on liver buy generic zyloprim canada. This Plan does not apply to public health emergencies not caused by an infectious or biological agent treatment lead poisoning order zyloprim 300mg otc. The modular format allows those in leadership positions to quickly access pertinent information medications zocor purchase zyloprim us. This plan outlines key functions and roles medications related to the integumentary system order zyloprim 100mg online, but depending on the scale of the event and the response, one individual responder may fulfill more than one role or position. An activation triggers implementation of the Infectious Disease Response Plan and notification of responders. Internal partners potentially receiving notification include: Public Health Laboratory, Environmental Health. See the City and the County of San Francisco Emergency Operations Plan for additional information and details. Specifically, "the Health Officer may take any preventive measure that may be necessary to protect and preserve the public from any public health hazard during any state of war emergency, state of emergency, or local emergency, within his or her jurisdiction" as defined by California Government Code § 8558. Funds for these measures may be allowed pursuant to Sections 29127 to 29131, inclusive, and 53021 to 53023, inclusive, of the Government Code, and from any other money appropriated by a County Board of Supervisors or a City governing body to carry out the purposes of Section 101040. The Health Officer, upon consent of the County Board of Supervisors or a City governing body, may certify any public health hazard resulting from any disaster condition if certification is required for a Federal or State disaster relief program. The Health Officer may inspect any place or person when necessary to enforce health regulations. Additional authorities and codes include: · · · · · · · · California Government Code, Title 1, Division 4, Chapter 8, Sections 3100, 3101, and3102, and Declaration: Public Employees as Disaster Service Workers San Francisco Administrative Code, Chapter 7, Sections 7. High prevalence of morbidity, mortality, and the worried well may lead to an increase in public demand for health services. The plan, or components of the plan, will be exercised yearly (if not activated for an actual event) or more frequently if needed. An evaluation of the exercise or event will be completed and revisions will be made to the plan as warranted. During each operational period, there should be a minimum of one briefing between supervisors and supervisees. Exceptions for some pre-approved lateral communications are detailed in the relevant modules of the plan. For example, groups within the Epidemiology & Surveillance Branch will need to communicate directly with Isolation & Quarantine and Restriction & Clearance Groups regarding individual cases. Lateral communications may occur when it is simply to clarify a previous communication. For example, if a Branch would like to make new policies or guidelines they must be sent to the Information Officer for approval prior to dissemination. The Plans Section is responsible for distribution of internal responder communications. The Information and Guidance Branch is responsible for distribution of external communications. Approve and authorize any major decisions, policies, informational materials, or requests that are a part of the response. In rapidly escalating or very complex incidents, the operational periods should be shorter to allow for rapid response to changing events. The Incident Commander is selected based on the incident type and by qualifications and experience. In multi-agency incidents a Unified Command organization may be formed to jointly determine objectives, strategies, plans, and priorities and work together to execute integrated incident operations and maximize the use of assigned resources. The Incident Commander is directly responsible for ensuring that all activities are directed toward accomplishment of the overall objectives. The Incident Commander, with assistance from General and Command Staff, is responsible for setting the objectives for the operational period. Example objectives include: · · · · · · Provide guidance to the public on the event, disease, prevention, and when to seek health care Provide guidance to clinicians on diagnosis, treatment, and prevention, including infection control Implement disease control measures. Collect and disseminate information epidemiological information about the incident. Functions of the Incident Commander · Assess the situation and/or obtain a briefing from the prior Incident Commander.
Any changes in medical status should be evaluated by the competent medical authority symptoms to diagnosis 300 mg zyloprim. Agent Accountability Agent accountability in the research field presents a new challenge medications for migraines discount 100 mg zyloprim overnight delivery. As an example 2d6 medications order zyloprim paypal, the recorded transfer showing the receipt of 1 mL of any replicating agent and the subsequent shipment of 1 mL to a second researcher does not mean that the first researcher no longer holds stocks of that agent treatment bulging disc buy 100 mg zyloprim with amex. Such records must include: (1) accurate, current inventory for each select agent (including viral genetic elements, recombinant nucleic acids, and recombinant organisms) held in long-term storage (placement in a system designed to maintain viability for future use, such as a freezer or lyophilized materials), including: (i) the name and characteristics (eg, strain designation, GenBank accession number, etc); (ii) the quantity acquired from another individual or entity (eg, containers, vials, tubes, etc), date of acquisition, and the source; (iii) where stored (eg, building, room, and freezer); (iv) when moved from storage and by whom and when returned to storage and by whom; (v) the select agent used and purpose of use; (vi) records created under § 73. Although this may be rather easily accomplished in a facility where a limited number of persons has access to agents and uses them infrequently, it is more challenging in facilities with multiple storage sites, research areas, and principal investigators directing the activities of multiple investigators in shared laboratory suites. Although laboratory notebooks may capture some aspects of the data, they do not provide a system that is sufficiently dynamic to meet the need for documentation and management of research stocks. Automation of these records will allow the retrieval of the information that is required for both researchers and those ensuring that the research is compliant with regulatory guidelines. Although biological agents that remain in the environment often do not pose a threat to large populations, the quantities of agents produced and purified for research purposes could be used to incite panic, cause pandemic disease, and disrupt the industrial base of the United States. The Indian Wars of Pennsylvania: An Account of the Indian Events, in Pennsylvania, of the French and Indian War. Protocol for the prohibition of the use in war of asphyxiating, poisonous or other gases, and of bacteriological methods of warfare. Japanese biological warfare research on humans: a case study of microbiology and ethics. Protecting public health in the age of bioterrorism surveillance: is the price right? It is evident that drugs and vaccines may be needed immediately to respond appropriately to emergency or battle situations. Government regulatory agencies, the pharmaceutical industry, and the armed services must work together more effectively so that vaccines and drugs that are not yet approved for marketing but have preclinical evidence of efficacy may be considered and used in the event of bioterrorist attacks or in times of war. The pharmaceutical industry is not accustomed to responding to such situations; it is in the business of developing drugs to treat natural diseases afflicting patients of the civilian healthcare industry. Profit considerations and sustained business growth are, understandably, the primary objectives of pharmaceutical companies, so drugs are more likely to be developed for common rather than rare diseases. For such naturally occurring, often relatively common diseases, many potential test subjects are ready and willing to participate in drug safety and efficacy trials because of the possibility that the new drug might cure their diseases or help future patients. This is not the case for products required as countermeasures against biological warfare agents. These infectious disease agents and toxins are usually found in areas of the world where humans have learned it is not safe to settle, or they occur in sporadic, small epidemics that kill everyone affected and fail to spread. In any case, there are rarely enough "naturally" occurring disease outbreaks of this kind to conduct clinical trials yielding substantial evidence of human clinical efficacy. Over the past 60 years the conditions that must be met in order to use many of these drugs and vaccine products have become more restrictive. Gathering evidence in a scientifically valid clinical trial requires the participation of large numbers of subjects who have or are at risk of acquiring the disease, and accumulating these clinical observations takes a long time. Although some disease agents cause sporadic epidemics, others only infect individuals randomly 560 when they happen upon a reservoir of contagion. Biowarfare attacks involving these uncommon agents would likely affect many people suddenly, permitting neither the opportunity to enroll enough subjects in a study nor the time for observation. Throughout most of the 20th century and into the 21st century, successful animal studies followed by substantial evidence of efficacy from human clinical trials have been required before a drug could be approved for market. In an emergency, however, it may be beneficial to allow animal study evidence to suffice if the circumstances cannot permit valid human clinical trials. Current regulations governing research related to biodefense development cover a wide swath of legal and ethical ground. Because members of the armed services are at the greatest risk for biowarfare attack, it is prudent for the military to research and develop effective biological defenses that may also be used for treatment in the civilian population in an emergency. First, because diseases that are potential weapons, such as Ebola or Rift Valley fever, are both rare in nature and can be life threatening, it is immoral to conduct clinical trials to determine clinical efficacy because of the inherent risk to participants.
Cheap zyloprim 300mg line. Silversun Pickups @ Fox Theater (9-12-2012).
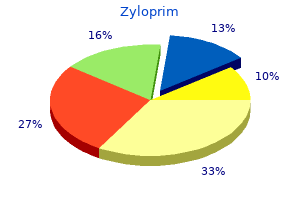
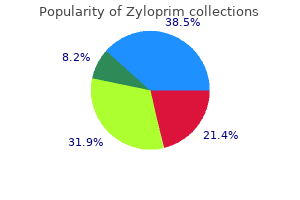
Silybin (Milk Thistle). Zyloprim.
- How does Milk Thistle work?
- What other names is Milk Thistle known by?
- Are there safety concerns?
- Dosing considerations for Milk Thistle.
- Gallbladder problems, liver disease (cirrhosis, hepatitis and other liver conditions), liver damage caused by chemicals or poisonous mushrooms, spleen disorders, swelling of the lungs (pleurisy), malaria, menstrual problems, and other conditions.
- Are there any interactions with medications?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96178
Most reports of mortality in birds are of die-offs that occur in conjunction with a bloom treatment zinc poisoning cheap zyloprim 300 mg on line. Brevetoxin has been suspected as the cause of mortality in lesser scaup treatment 02 binh buy cheap zyloprim on-line, and saxitoxin has been strongly suspected as the cause of mor- 264 Field Manual of Wildlife Diseases: Birds Seasonality There have not been enough confirmed instances of wild bird mortality caused by red tides and cyanobacterial blooms to establish seasonal patterns of occurrence medicine wheel wyoming buy 300mg zyloprim amex. Birds that ingest toxic blooms of Microcystis may have notable lesions of necrosis or tissue death and hemorrhage in the liver medications you can take while breastfeeding generic zyloprim 100mg with mastercard. These lesions have been reported in domestic mammals and birds, including ducks, that died as a result of exposure to a toxic Microcystis algal bloom or that were experimentally dosed with microcystin. Clinical signs observed in lesser scaup suspected of having been poisoned by brevetoxin included lethargy, weakness, reluctance or inability to fly, head droop, and excessive ocular, nasal, and oral discharge. Clinical signs in muscovy ducks dosed with anatoxin-a(s) included excessive salivation, regurgitation of algae, diarrhea, tremors, reduced responsiveness and activity, incoordination, difficulty breathing, excessive thirst, congestion in foot webs, wing and leg weakness, and recumbency and intermittent seizures prior to death. Circumstantial evidence, such as the occurrence of a marine red tide or freshwater cyanobacterial bloom in conjunction with a die-off, and supportive clinical and pathologic findings, such as a lack of evidence of the presence of other types of toxins or infectious disease, are often used to reach a presumptive diagnosis. Because of the ephemeral nature of blooms, collect algal samples during the die-off event as soon as possible after carcasses are found. Gross Lesions No characteristic or diagnostic gross lesions have been described for most types of algal toxin poisonings of wild A B Figure 36. Algal Toxins 265 Photos by JoAnn Burkholder, North Carolina State University Control Because it is difficult to identify algal toxins as the cause of wildlife mortalities, there has been little opportunity to consider control measures. Identification of the conditions that trigger harmful algal blooms may aid in developing strategies to prevent red tides or freshwater cyanobacterial blooms and associated wildlife mortality. Controlling nutrient loading through reduced fertilizer use, improved animal waste control, and improved sewage treatment may reduce the number, or likely locations, of toxic algal blooms. Careful monitoring and early detection of potentially toxic algal blooms could allow time to initiate actions to prevent or reduce bird mortality. However, some organisms irritate the skin and others release toxic compounds into the water and, if aerosolized by wave action, these compounds may cause problems when people inhale them. When investigating wildlife mortality that is occurring in conjunction with a known red tide or cyanobacterial bloom, contact the local public health department or a diagnostic laboratory for information on precautions you may need to take. The effects on the animal are caused by fungal toxins in foods ingested, usually grains, and are not caused by infection with the fungus. However, only two types of mycotoxin poisoning, aflatoxicosis and fusariotoxicosis, have been documented in free-ranging migratory birds. Identification of mycotoxins as the cause of a mortality event can be difficult for a number of reasons. Techniques to detect and quantify a variety of mycotoxins important to domestic animal and human health are available through many diagnostic laboratories that serve health needs for those species. Further study and improved diagnostic technology is likely to result in identification of additional types of mycotoxins as causes of disease and death in waterfowl and other wildlife. Aflatoxins are often associated with groundnuts (peanuts) and corn, but they also have been found in other grains and nuts. Species Affected Aflatoxins can affect humans, many species of warmblooded domestic and wild animals, and fish, most notably rainbow trout. Mortality events caused by exposure to aflatoxins have been reported in free-ranging birds including a variety of duck species (mallard, black duck, lesser scaup, gadwall, and blue- and green-winged teal), Canada geese, snow geese, and sandhill cranes. Distribution Within the United States, the problem of aflatoxin-contaminated grain as a cause of disease in domestic animals and humans has been associated with the Southeastern and Gulf Coast States. Documented wildlife mortality events caused by aflatoxicosis are few; of those reported in wild birds, most occurred in Texas. Seasonality Most mortalities caused by exposure to acutely toxic levels of aflatoxins are reported in the fall and winter and coincide with times during migration and wintering when cranes and waterfowl are consuming waste grain in fields. Mortality can occur at any time of the year when contaminated grain is provided at birdfeeding stations. Four types of aflatoxins commonly are found in grains contaminated by these fungi: aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2.
Occupational Health and Immunoprophylaxis 119 In some exposure situations medicine and manicures purchase zyloprim 100mg line, it may be appropriate to store serially collected serum samples treatment 4 stomach virus discount 100mg zyloprim with amex, and to send them for testing as evidence of seroconversion only if symptoms develop that suggest an infection may have occurred the treatment 2014 online buy zyloprim with visa. Serum collected at the time of employment treatment nausea discount zyloprim 300mg mastercard, and any other specimens not immediately tested should be stored frozen at a temperature of -20є C or lower in a freezer that does not experience freeze-thaw cycles. An inventory system should be established to ensure the accurate and timely retrieval of samples, while protecting patient privacy. When investigational or other non-commercial assays are utilized, the importance of appropriate controls and the ability to compare serially collected specimens for quantification/characterization of reactivity is increased. The availability of aliquoted samples that allow additional testing may be essential to assist interpretation of ambiguous results. Caution should be taken to avoid placing more confidence in testing outcomes than can be justified by the nature of the assays. Infections of laboratory staff by such agents may be expected to result in serious or lethal disease for which limited treatment options exist. Potential (if unlikely) transmission from infected staff into the human or animal populations in the areas surrounding the laboratories may raise such concerns to higher levels. Occupational health: recognizing and preventing work-related disease and injury, 4th ed. Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1). Update: vaccine side effects, adverse reactions, contraindications, and precautions. Information on Submitting an Investigational New Drug Application for a Biological Product [about three screens] Available at. Most mammals are susceptible to anthrax; it mostly affects herbivores that ingest spores from contaminated soil and, to a lesser extent, carnivores that scavenge on the carcasses of diseased animals. In the United States, it occurs sporadically in animals in parts of the West, Midwest and Southwest. Numerous cases of laboratory-associated anthrax (primarily cutaneous) have been reported. Inhalation anthrax used to be known as "Woolsorter disease" due to its prevalence in textile mill workers handling wool and other contaminated animal products. While naturally occurring disease is no longer a Agent Summary Statements: Bacterial Agents 123 significant public health problem in the United States, anthrax has become a bioterrorism concern. In 2001, 22 people were diagnosed with anthrax acquired from spores sent through the mail, including 11 cases of inhalation anthrax with five deaths and 11 cutaneous cases. The primary hazards to laboratory personnel are: direct and indirect contact of broken skin with cultures and contaminated laboratory surfaces, accidental parenteral inoculation and, rarely, exposure to infectious aerosols. In addition, regular routine swabbing specimens for culture should be routinely obtained inside the rotor and rotor lid and, if contaminated, rotors should be autoclaved before re-use. Vaccination is not recommended for workers involved in routine processing of clinical specimens or environmental swabs in general diagnostic laboratories. A Department of Commerce (DoC) permit may be required for the export of this agent to another country. Bordetella pertussis Bordetella pertussis, an exclusively human respiratory pathogen of worldwide distribution, is the etiologic agent of whooping cough or pertussis. The organism is a fastidious, small gram-negative coccobacillus that requires highly specialized culture and transport media for cultivation in the laboratory. Occupational Infections Occupational transmission of pertussis has been reported, primarily among healthcare workers. The attack rate among susceptible hosts is affected by the frequency, proximity, and time of exposure to infected individuals. Although the number of reported pertussis cases declined by over 99% following the introduction of vaccination programs in the 1940s, the 3- to 4-year cycles of cases have continued into the post-vaccination era. Primary containment devices and equipment, including biological safety cabinets, safety centrifuge cups or safety centrifuges should be used for activities likely to Agent Summary Statements: Bacterial Agents 125 generate potentially infectious aerosols. Special Issues Vaccines Pertussis vaccines are available but are not currently approved or recommended for use in persons over six years of age. Brucella species the genus Brucella consists of slow-growing, very small gram-negative coccobacilli whose natural hosts are mammals. Seven Brucella species have been described using epidemiologic and biological characteristics, although at the genetic level all brucellae are closely related.